BP Endo
Life Sciences
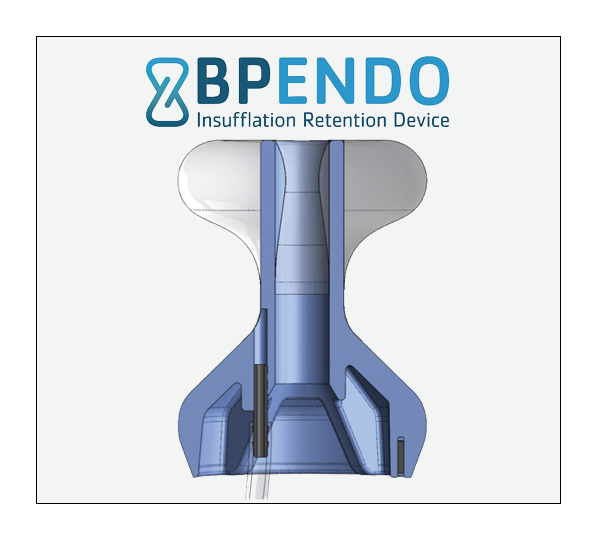
BP Endo’s one-of-a-kind Insufflation Retention Device (IRD) is an add-on device that solves the problem of air or fluid incontinence in colonoscopies by creating a seal in the rectum while allowing full freedom of movement for the endoscope.
Why we invested:
Market Need
A colonoscopy is an important procedure for screening for colon cancer, removing polyps, and controlling lower gastrointestinal bleeding. If air or fluid incontinence occurs during the procedure, the exam cannot be performed successfully. This is a regularly occurring problem, as an OU Medical School study recorded air incontinence occurring 24% of the time. This problem is estimated to cost the average gastroenterologist approximately $15,000 annually.

Founder & Team
Founder and CEO Dr. Robert Holbrook is a practicing gastroenterologist with 19 years of experience and more than 20,000 colonoscopies performed. Clinical Researcher Dr. Mohammad Madhoun is the local Site Director for the Gastroenterology Fellowship program while also serving as the Director of Endoscopy at the VA Medical Center.
-
"We could not be more pleased to have Boyd Street Ventures as an investor. Their management team and Strategic Advisory Board can be a great source of insight, particularly in the areas of strategy and operations, and they are actively engaged in the honing for success."
Jesse McCool Co-Founder & CEO, Wheeler Bio -
"James has been extremely helpful in making important introductions for us. I appreciate the fact that he’s always asking me about our plans to grow even faster, and it seems that every time I talk to him he has another great idea for a potential new customer."
Nathan Den Herder Founder & CEO, Ardley
